Case Study: The Metallurgy Within a Reheating Furnace at DanSteel
In this article, a team of researchers describe the technical, technological, and metallurgical characteristics in heating large-sized continuous cast slabs made of low carbon microalloyed steels, using the operation at DanSteel’s rolling complex 4200 as a case study. These characteristics ensure high quality heating process of slabs used for production of high-quality heavy plates weighing up to 63 tonnes*, which are particularly in demand in the offshore wind energy and bridge construction industries.
On the research team are the following: Eugene Goli-Oglu, Sergey Mezinov and Andrei Filatov, all of NLMK DanSteel, and Pietro della Putta and Jimmy Fabro of SMS group S.p.A.
This informative piece was first released in Heat Treat Today’s December 2024 Medical & Energy Heat Treat print edition.
*1 metric ton = 2204.6 pounds
The production of structural heavy plate steel is a complex multi-step process, the technological steps and operations of which have an impact on product quality and production economics. Slab reheating for rolling is one of the key process steps in the technological chain, directly linked to the quality and cost efficiency of heavy plate production process.
At DanSteel’s rolling complex 42001, continuously casted (CC) slabs are heated either in pusher type furnaces or walking beam furnaces depending on their cross section. In the case of big-size and heavy tonnage slabs with a cross-section of H x B up to 400 x 2800 mm, heating takes place in the latest generation of the SMS group walking-beam reheating furnace, installed in 2022. The main objectives of the installation of the new reheating furnace were the expansion of the product range towards the production of XXL high-quality heavy plates weighing up to 63 tonnes, which are most in demand in the offshore wind power and bridge construction industries, as well as improving the quality, economic, and environmental parameters of slab reheating process.

1 — steel with low titanium additions (Ti/N=3.24)
2 — steel with 0.02% Nb and Ti (Ti/N=3.33)
3 — steel with increased titanium content Ti/N=4.55
The aim of this article is to describe the technical, technological, and metallurgical characteristics in heating large-sized continuous cast slabs made of low carbon microalloyed steels and how this looks at the DanSteel’s rolling complex 4200.
Metallurgical Characteristics of Slab Heating
Heating of low carbon microalloyed steel slabs is one of the key technological steps in forming the optimal microstructural condition of heavy plates and their surface quality. In conjunction with microalloying, the technological parameters of heating affect such important characteristics as average grain size and uniformity of the austenitic structure, the composition of the solid solution and the type/thickness of the surface scale. In terms of heavy plate quality, the main realized task at the reheating stage is to obtain at the exit a slab with a setup temperature, the minimum temperature gradient along the thickness, width and length of the slab, optimal quality and quantitative condition of the surface scale.
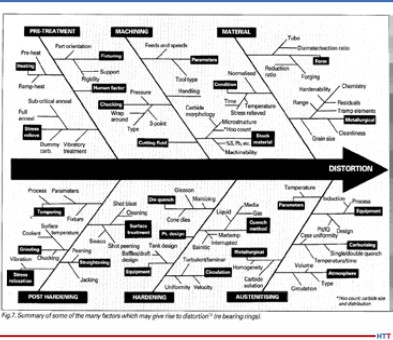
The heating temperature and its uniformity are important to form a microstructure of increased uniformity. It is known2 that a fine-grained austenitic steel structure has an increased grain boundary surface per volume unit, which leads to an excess of free energy of the system, which creates a driving force that determines the subsequent grain growth. The austenitic grain grows exponentially when heated in certain temperature ranges and this grain growth tendency is always present in low carbon microalloyed steels.

There are two general mechanisms of austenitic grain growth when heating slabs: normal and abnormal growth. That is, when reaching a certain temperature, which depends on the chemical composition, the austenite grain begins to increase very rapidly in apparent diameter. Abnormal grain growth can be observed in austenitizing steels containing strong CN-forming elements. Anomalous grain growth is not observed in simple low alloyed Si-Mn steels but at heating temperatures of 2102°F–2192°F, the grain grows to very large sizes (200 μm and larger).3
To avoid exponential grain growth of austenite during heating for rolling, dispersed particles that inhibit grain boundary migration are effectively used.4 The undissolved particles inhibit the migration of grain boundaries and thus inhibit the growth of austenitic grains. The nature of the release of particles and their effect on the average size of the austenitic grains of Ti and Nb alloyed low carbon steels is shown in Figure 1. It is important that the slab at the exit of the furnace has a given heating temperature without gradient limit deviations.
The main microalloying elements that form the optimal (fine grain) austenite structure as a result of the solid-solution effect and the formation of nitrides and carbides during slab heating are titanium, niobium, and vanadium (Figure 2).5 Titanium forms nitrides, which are stable at high temperatures in the austenitic range and allow control of the austenite grain size during heating before hot deformation. The binding of free nitrogen (which has a high affinity for carbide forming elements) by titanium has a positive effect on steel ductility and makes niobium more effective. Niobium is an effective microalloying element for refining the austenite grain during heating for rolling.6 It also has the positive effect of inhibiting austenite recrystallization during thermomechanical rolling.7
It is worth noting a number of works8, 9, 10, in which it was shown that increasing the heating temperature of V-Ti-Nb steel and the associated austenite grain enlargement does not significantly affect the size of the recrystallized grain, formed in the temperature range of complete recrystallization after repeated deformation under the same temperature and deformation conditions. This experimental result at first sight contradicts most recrystallization models11, 12, according to which the size of recrystallized austenite grain depends on the initial (before deformation) grain size and deformation temperature.

The microstructure and mechanical properties of the finished product directly depend on the heating temperature and are determined by the size and homogeneity of the austenitic grains, the stability of the austenite itself, influencing the condition of the excess phase and, consequently, the kinetics of its subsequent transformation. For timely recrystallization processes and control of dispersion hardening, it is necessary to balance the uniform fine grained austenitic microstructure and the transition of dissolved particles into solid solution when defining the heating temperature. Also, the heating temperature must be sufficiently high to fully undergo recrystallization in the interdeformation pauses.13 It should also be considered the possible negative phenomena of local and general overheating that occur when heating a slab above a certain temperature for a given steel and lead to a sharp increase in the austenitic grain size. The decreased heating temperature allows for a number of technological advantages: The possibility of reducing the pause time for cooling before the finishing step of rolling, increasing productivity of furnaces due to reduced heating time for rolling, and therefore the mill as a whole, as well as reducing the cost of the product due to saving fuel and reducing losses on scale. However, it should be remembered that some groups of low carbon steels have an optimal temperature range for heating, target temperatures above or below, which increase the heterogeneity of the microstructure. Thus, ensuring uniform heating to a given holding temperature and discharging slabs from the reheating furnace for subsequent rolling is an important technological task and contributes to the formation of austenitic microstructure and solid solution state of low carbon microalloyed steel with increased uniformity.
DanSteel Walking Beam Reheating Furnace
In 2022, DanSteel and SMS commissioned a new walking-beam reheating furnace (Figure 3) with a design capacity of up to 100 tonnes/hour, expanding the range of slabs heated to a maximum cross section of H × B 400 × 2800 mm and improving heating quality. The maximum temperature difference between the coldest and the hottest points on the slabs is not more than 30°C. The new furnace has been designed with a focus on environmental and energy efficiency and has reduced CO2 emissions by 17–18% compared to the furnaces already in operation in the plant.

The walking beam reheating furnace is for heating cast carbon, low-carbon, and low-alloy steel slabs weighing up to 63 tonnes. The main production characteristics of the furnace as part of DanSteel 4200 rolling complex are shown in Table 1.

Slabs are moved through the furnace by moving the walking beam in four steps: lifting, moving forward, lowering below the level of the fixed beams, and moving the walking beams backwards. The speed of the slab moving in the furnace is controlled by changing the movement intervals between the movement cycles of the beams and depends on the variety of heated slabs. Slab discharging from the furnace is carried out shock-free, using a special machine that moves the slabs from the furnace beams to the mill roller conveyor. The furnace is equipped with a modern automated process control system and a system of instrumentation and sensors that allows the heating of steel without the direct involvement of technical personnel and provides for the measurement, regulation, control, and recording of all operating parameters.
The furnace type is reheating, walking beam, regenerative, multi-zone, double-row, double-sided heating, frontal charging, and discharging furnace. The furnace is designed for natural gas operation with the possibility of a quick conversion, within three weeks, of up to 40% of the capacity for hydrogen operation. The conversion is carried out by means of a minor modernization of the burner’s inner circuit, the installation of hydrogen storage auxiliary equipment and the regulation of the hydrogen supply to the modified nozzles. It is planned that the replacement of natural gas by hydrogen will also reduce the consumption of natural gas by ~40% and hence reduce the negative impact of the process on the environment. Feeding control as well as optimum pressure is controlled by a special automated control system. Table 2 shows the main technical characteristics of the furnace.

The air is heated in a metal recuperator, located on the furnace roof. The combustion products pass between the tube and the air passes through the recuperator tubes. The air is blown by a blower into the recuperator and transported to the burners through thermally insulated air ducts. The gas and air from the common pipelines are supplied to each zone via zone headers, on which flow meters and actuators for flow controllers are installed to ensure an ideal furnace atmosphere with an O2 content of about 0.7–1.0 %.
The furnace has 6 heating zones, 3 upper and 3 lower, with 24 SMS-ZeroFlameTM burners (Figure 4a) for ultra-low nitrogen oxide concentrations and high thermal efficiency.14 The burners consist of a metal casing with external cladding for heat protection, several fuel and combustion air lines, a pre-combustion chamber and an air deflector made of refractory material with high alumina content.

The particular design of the installed burners allows them to operate using three modes:
- Flame mode (Figure 4b), used for ignition and at low temperature, but even then, the NOx level remains low thanks to the triple-stage air supply
- Flameless mode (“aka invisible flame,” Figure 4c), which ensures high slab heating uniformity over the cross section creating a homogeneous, invisible flame with minimum NOx emissions
- Mixed “booster” mode, allowing a 15% to 20% increase in nominal heat input, and a rapid increase in zone temperature if the furnace setting is changed due to a change in steel grade or increased capacity

The combustion gases from the gas combustion heat the metal through direct radiant heat transfer, as do the combustion gases heat the burner units, the furnace roof and walls, which in turn heat the slabs in the furnace through indirect radiant heat transfer. The optimum combination of burner arrangements ensures intensive and uniform heating. The mutual movement of combustion gases and metal is counter current. Combustion gases from the recuperation zone are conveyed by a waste gas duct to the heat exchanger (where they heat the air) and then through a waste gas intake to the chimney and exhausted to the atmosphere. The rotating valve is installed in the exhaust duct between the recuperator and the chimney and is used to control the pressure in the heater.

The skids are cooled by chemically treated water, which circulates in a closed circuit. A dry fan cooling tower is used to dissipate the heat from the cooling water. Steel is charged into the furnace by a charging machine that moves the slabs from the charging roller table to the furnace skids.
Technical Features of Slab Heating
The highly even heating of slabs in furnace 3 of DanSteel is ensured by the optimum arrangement of the burners, flameless fuel combustion, triple skids shift, and warm riders on the skids. The evenness of the slab heating corresponds to a maximum temperature difference in the longitudinal section of up to 20°C, and the maximum difference between the coldest and hottest points of the slab must not exceed 30°C.

Earlier in work15, it was shown that when heating a 250 mm slab in the old furnace no. 2, the maximum temperature gradient was for a long time within 250-300°C, and at the exit of the furnace the slab had a sensitive temperature difference in cross section. Figure 5 shows an industrial schedule of heating slabs cross-section 250 x 2800 mm in the new furnace no. 3. Analyzing thermal and technical data of slab heating for heavy plate production using the new furnace, it should be noted that the slab temperature uniformity distribution during the whole heating period is essential. When heating slab cross-sections 250 x 2800 mm in the new furnace, the maximum temperature gradient does not exceed 130°C (Figure 5). The peak values of temperature gradients are situational in nature and appear only for a short period of time and at times of adaptation of the control model of heating for each specific slab in the active zones of the furnace. For slabs with a thickness of 250 mm the most critical time is the time interval between approx. 90 and 120 minutes during which the upper and lower surfaces of the slab are actively heated. During the last 20 minutes in the soaking and equalizing phase, the temperatures at ¼, ½, and ¾ of the slab thickness reach a maximum gradient of no more than 20°C. As can be seen from the graph in Figure 5, heating of 250 x 2800 mm slabs to a given temperature of 1150°C takes no more than 4.5 hours. It is possible to reduce the heating time, however, with a certain decreasing of quality.

A similar schedule for heating 400 x 2800 mm slabs is shown at Figure 6. For large cross-section slabs with a thickness of 400 mm, the heating time is in the range of 9–10 hours. The heating time can be reduced to 8 hours, but also with a decrease in the quality of heating towards an increase in the temperature gradient across the thickness of the slab. It should be noted that the temperature increases smoothly in the heating curves at ¼, ½, and ¾ of the slab thickness. From the peaks of the upper furnace temperature curve, the discreteness of the adaptation adjustments of the furnace heating control model can be evaluated.
Heavy Plate Temperature Profile
The DanSteel 4200 Rolling Complex is equipped with twelve control pyrometers and three thermo scanners that measure the temperature of 100% of the top surface of the plate at reference points in the heavy plate production process. The data obtained can be used to accurately and in real time evaluate the temperature uniformity of the plate in width and length direction.

As an example, Figure 7 shows the results of a scan of the surface temperature of 120 mm thick rolled steel heavy plate after deformation stage is completed and before the start of final cooling in an accelerated cooling unit. Two states of temperature gradients occurring during production are considered: uneven heating and uniform heating. Figure 7a shows the temperature field of a plate with expressed temperature irregularity. The main reason for the marked irregularity in the temperature field of the rolled plate is non-optimal modes of heating of the slab. It can be seen that the central part of the plate has the temperature specified by the technology, while the head and tail overheated by 50-60° C relative to the specified temperature at a maximum permissible deviation of not more than 30°C. Figure 7b shows the temperature field of a plate with a high degree of uniformity. Approximately 95% of the surface of such a plate is at the process-specified temperature with a deviation of ±3°C. The maximum temperature gradient does not exceed 10°C.
The temperature profiles of the top (Figure 7c and Figure 7d) and bottom (Figure 7d and Figure 7e) rolled surfaces, obtained from control pyrometers, show that the nature of the temperature non uniformity is repeated on the upper and lower surfaces of the plate. In the first “non-optimal” case the temperature gradient of the top surface reaches about 76°C, and on the bottom surface: -54°C. In the case of uniform heating, the gradient of the top surface of the plate does not exceed 3–6°C and the bottom surface: 5–11°C.
Preventive Maintenance System
The DanSteel new walking beam furnace is also equipped with an innovative maintenance support tool named SMS Prometheus PMS (Preventive Maintenance System). It consists of a software platform collecting and elaborating the data provided by an extended number of sensors strategically placed over several mechanical components of the furnace, with the goal of predicting possible malfunctioning. The monitored equipment includes the key handling devices, like the slab charger, the slab extractor or the walking beam system, as well as the hot air recuperator, the combustion air fans of the main components of the water treatment fan. The software algorithm is able to extrapolate some data from the sensor measurements to assess the key performance trends of the related component and anticipate the necessity of intervention for maintenance or repair before any actual damage happens.

In the example of Figure 8, the trends are shown that correlate the walking beam movement and the cylinders pressure to the slab load inside the furnace. Any significant deviation in respect to the foreseen pattern denotes a movement anomaly and will trigger a notification to the control system, that allows the plant maintenance team to act preventively in view of a potential failure.
Conclusion
A new walking-beam reheating furnace with a designed productivity of up to 100 t/h was put into operation at DanSteel rolling complex 4200. This allowed expanding the range of heated large-size slabs with a maximum cross-section of H x B 400 x 2800 mm and weighing up to 63 tonnes. The implemented project has provided increased uniformity of heating along the thickness, width and length of slabs with average maximum values of temperature gradients in the three directions not exceeding 30°С (80°F) and reduced consumption of natural gas to the level of 31–32 m3/t of finished product. More uniform heating of slabs ensured improved temperature field uniformity of rolled heavy plates. The constructive possibility of a partial transition to the use of hydrogen instead of natural gas was taken into account.
References
- I. Sarkits, Y. Bokachev, E. Goli-Oglu, “Production of heavy plates on the rolling mill 4200 DanSteel A/S,” Stahl und Eisen. 2014. no. 4, 57–61.
- Imao Tamura, Hiroshi Sekine, Tomo Tanaka, Chiaki Ouchi, Thermomechanical Processing of High-strength Low-alloy Steels (Butterworth-Heinemann, 2013), 256.
- Antonio Augusto Gorni and José Herbert Dolabela da Silveira, “Accelerated Cooling of Steel Plates: The Time Has Come,” Journal of ASTM International 5, no. 8 (2008): 358–365.
- Y. I. Matrosov, “Complex microalloying of low-pearlite steels subjected to controlled rolling,” Met Sci Heat Treat No. 28 (1986): 173–180.
- S. V. Subramanian,, G. Zhu, C. Klinkenberg, K. Hulka, “Ultra Fine Grain Size by Dynamic Recrystallization in Strip Rolling of Nb Microalloyed Steel,” In Materials Science Forum. Vols. 475–479 (2005): 141–144.
- S.C. Hong, S. H. Lim, “Inhibition of Abnormal Grain Growth during Isothermal Holding after Heavy Deformation in Nb Steel,” ISIJ International 42, no. 12 (2002): 1461–1467.
- K. Hulka, A. Kern, U. Schriever, “Application of Niobium in Quenched and Tempered High-Strength Steels,” Materials Science Forum vols. 500–501 (2005): 519-526.
- C. M. Sellars, J. A. Whiteman, “Recrystallization and Grain Growth in Hot Rolling,” Metal Science no. 13 (1979): 87–194.
- H. Tamehiro, N. Yamada, H. Matsuda, “Effect of the Thermo-Mechanical Control Process on the Properties of High-strength Low Alloy Steel,” Transactions of the Iron and Steel Institute of Japan Vol. 25, Issue 1 (1985): 54–61.
- Sh. Liang, F. Fazeli, H. S. Zurob, “Effects of solutes and temperature on high-temperature deformation and subsequent recovery in hot-rolled low alloy steels,” Materials Science and Engineering A., vol. 765 (2019): 138324.
- H. Yada, “Prediction of Microstructural Changes and Mechanical Properties in Hot Strip Rolling,” Proceeding of the International Symposium on Accelerated Cooling of Rolled Steel. Winnipeg, Canada. 1988. 105-119.
- W. Roberts, A. Sandberg, T. Siweski, T. Werlefors, “Prediction of Microstructure Development during Recrystallization Hot Rolling on Ti-V-steels,” ASM HSLA Steels Technology and Applications Conference. Philadelphia, USA. 1983. 35–52.
- R. Wang, C. I. Garcia, M. Hua, K. Cho, H. Zhang, A. J. Deardo, “Microstructure and precipitation behavior of Nb, Ti complex microalloyed steel produced by compact strip processing,” ISIJ international 46, no. 9 (2006): 1345-1353.
- “Innovation in combustion process,” SMS group, https://www.sms-group.com/en-gb/insights/all-insights/innovation in-combustion-process (date of review 2023-03-20).
- V. A. Tretyakov, Bokachev, A. Yu, A. N. Filatov, E. A. Goli-Oglu, Development of a digital twin of the process of controlled rolling of thick plate from high-strength low-alloy steels. Message 1. Simulation of slab reheating in continuous furnace with a prediction of austenite grain size before rolling. // Problems of ferrous metallurgy and materials science. 2022. no. 2, P. 30-40.
This article content is used with permission by Heat Treat Today’s media partner Furnaces International, which published this article in September 2023.
About the Authors:

Head of Product Development, Technology and Technical Sales Support
NLMK DanSteel

Metallurgist
Product Development and Technical Sales Support
NLMK DanSteel

Vice-President
Reheating and Heat Treatment Plants
SMS group S.p.A.

Head of the Technical Department – Furnace Division
SMS group S.p.A.
Eugene Goli-Oglu has worked at NLMK DanSteel since 2013 and has led Product Development, Technology and Technical Sales Support functions for steel heavy plate production. Eugene received his Master degree in Metal Forming in 2007, a second Master’s degree in Economy in 2009, and a PhD in Metallurgy and Thermal Processing of Metals and Alloys in 2012. He has authored/co-authored 90+ publications in technical journals.
Sergey Mezinov has worked at NLMK DanSteel since 2007 as an engineer of the Project Department and process engineer of the Quality Department. In 1995, Sergey graduated as an heat-power engineer. He has authored/co-authored of 2+ publications in technical journals and authored/co-authored two patents.
Andrei Filatov has worked at NLMK DanSteel since 2019 as a metallurgist in the Product Development and Technical Sales Support department. In 2015, Andrei graduated as an engineer physicist, and in 2019, he completed postgraduate studies in Metallurgy and Thermal Processing of Metals and Alloys. He has authored/co-authored 20+ publications in technical journals.
Pietro della Putta is the vice president of the Reheating and Heat Treatment Plants department at SMS group S.p.A. Jimmy Fabro is the head of the Technical Department – Furnace Division at SMS group S.p.A.
Jimmy Fabro is the head of the Technical Department – Furnace Division at SMS group S.p.A.
Find heat treating products and services when you search on Heat Treat Buyers Guide.Com
Case Study: The Metallurgy Within a Reheating Furnace at DanSteel Read More »























 Keenan Cokain, global sales and applications coordinator and Michael Cochran, an applications engineer, both from Pittsburgh’s Bloom Engineering, an industrial combustion and controls company, add another consideration: “Natural gas is a vital primary energy source globally and will likely remain so over the next 10 years. Although energy demands will likely show an overall decline in 2020, over the next 10 years, global natural gas consumption will likely rise as it continues to grow in comparison to other fossil fuels (such as oil and coal) as a percentage of the global primary energy consumed.
Keenan Cokain, global sales and applications coordinator and Michael Cochran, an applications engineer, both from Pittsburgh’s Bloom Engineering, an industrial combustion and controls company, add another consideration: “Natural gas is a vital primary energy source globally and will likely remain so over the next 10 years. Although energy demands will likely show an overall decline in 2020, over the next 10 years, global natural gas consumption will likely rise as it continues to grow in comparison to other fossil fuels (such as oil and coal) as a percentage of the global primary energy consumed.