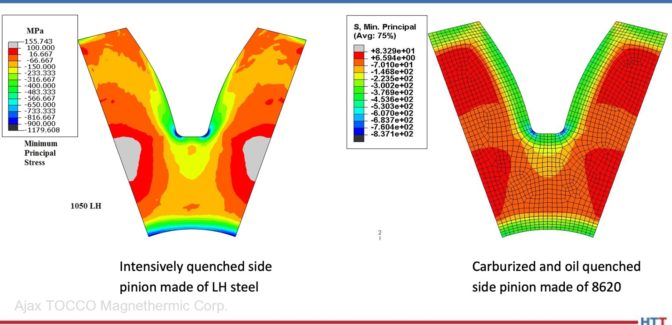
Heat Treat Radio #79: All Things Auto Industry Quenching with Scott MacKenzie
Heat Treat Radio host and Heat Treat Today publisher, Doug Glenn, sits down with Dr. D. Scott MacKenzie, the senior research scientist and metallurgist at Quaker Houghton, for a deep dive into quenching in the automotive heat treat industry. We’re talking the implications of electric vehicles (EV), aluminum and automotive manufacturing, simulation, and training in quench and heat treat.
This automotive industry-focused episode about quenching comes on the heels of Heat Treat Today's August 2022 Automotive print edition.
Below, you can watch the video, listen to the podcast by clicking on the audio play button, or read an edited transcript.
The following transcript has been edited for your reading enjoyment.
Doug Glenn (DG): We’re here today with Dr. D. Scott MacKenzie from Quaker Houghton. We’re going to talk a little bit about quenching. Scott, first off, welcome to Heat Treat Radio.
Scott Mackenzie: Thank you. And I just go by “Scott.”

DG: Very good. You and I have known each other long enough, I can probably do that and get away with it, so that’s okay.
SM: Everybody calls me Scott. I don’t like being called doctor.
DG: Let me give the folks a bit of an intro and then I’m going kind of highlight some of the stuff we’re going to be covering today. We’re going to be talking quenching because Scott is obviously the “quench king” here. We’re going to talk about EV (electric vehicles) a little bit. We’re going to talk about aluminum in the automotive industry, modeling and simulation and, briefly, we’re going to talk about a product that Quaker Houghton came out with not too terribly long ago called GREENLIGHT. We’re also going to talk about training for captive and/or commercial heat treaters in regard to quenching. So, that’s stuff to look forward to.
First, let me just mention that Scott is presently the senior research scientist and metallurgist for Quaker Houghton (formerly Houghton International) in Conshohocken, PA. He joined Houghton International in 2001 as a technical specialist heat treating marketing and moved into the heat treat laboratory, to the supervisor position, in 2007. Prior to joining Houghton, he worked as an associate technical Fellow in failure analysis, at the company actually, for six years and manufacturing engineer for the steel and aluminum heat treating departments for twelve years. He was past president of IFHTSE (International Federation for Heat Treatment and Surface Engineering) from 2018 to 2020. He is an active member of ASM and served on a lot of committees at ASM as well as member or chairman. You’ve authored, Scott, several books and over one hundred peer-reviewed papers.
So, I expect to see an increase in induction hardening or, at least, stay the same, but more atmosphere, traditional atmosphere, endothermic atmosphere and quenching and quenching in a quenchant — that’s going to be drastically hit in the next five to ten years.
Scott got his BS in metallurgical engineering from Ohio State University and got his MS and PhD from the University of Missouri Rolla. Bottom line, Scott is well qualified to talk about quenching and that’s what we want to do.
Scott, before we jump in and ask the first question, is there anything else you’d like to share with us about your background: where you’ve been, some of your more interesting experiences, or things that would be of interest?
SM: One, I got my PhD late in life. I started on my PhD when I was 45. So, I already had practically 15 years of experience on the shop floor, mostly doing heat treat with doing all the landing gear for the F/A-18, the F15, the AV-8B Harrier, wing skins for aircrafts like MD-80, DC-9, DC-10, MD-11 and then later when I was at Boeing, some of the 737 wing skins and all that sort of stuff. A lot of manufacturing on the shop floor.
DG: It’s a real advantage going to school late in life, too, because you come there with a real different perspective. You’re not green, you know the questions to ask, you know what’s BS and what’s not BS.
SM: Well, the trouble with that is twofold: One, you’re not willing to take any BS from the professors, right? And also, you are more willing to challenge them. In that, from a teacher’s perspective, you’re a much more difficult student because you question more. But, by the same token, you’re also easier to teach because you’re more motivated — you’re not just there because mommy is paying the bill.

DG: Yes, absolutely. I taught school a little bit, not college level, but I’d much rather have students that are engaged.
Let’s talk about electric vehicles. It’s a transition that seems to be coming on. Let’s talk about it in terms of heat treating, in general, and quenching, in particular. What do you think about this EV thing? How is it going to impact heat treat?
SM: Well, there’s a big thing about EV that is going to drastically impact heat treating and the heat treating industry, as well as quenchants. Presently, approximately 50% of the heat treaters, (at least in the U.S. and probably globally), are related to heat treating of gears. . . transmission gears, etc. Then we have doing other suspension components, like the tulips with the drive shaft, etc. But should the complete EV — and I’m not talking hybrids, I’m talking about a complete EV . . . EV’s drive by, you put your foot on the accelerator, it goes through, like, a potentiometer computer and that will control the four motors at each wheel, or just two. There’s no transmission involved. So, since there’s no transmission involved, there is no requirement for gears and since there is no requirement for the gears, there is no requirement for heat treat. And so, if we get a full implementation of electric vehicles, we’ll have roughly 50% excess capacity in the heat treat industry, which means the grid people won’t be selling as many grids and the quenchant people won’t be selling as much quenchant.
Even in the racing world — why, even Formula 1 is going to electric, they have Formula E which is all electric. You look at even the super cars. Aston Martin just announced a fully electric vehicle. Pagami just came out with a [indiscernible] last night. (I’m a big fan of Aston Martin.) You have the Lamborghini, Ferrari – they’re all coming out with electric vehicles, either hybrid or fully electric. Volvo is committed to 100% electric by 2025. So, we need to pay attention to where the industry is going.
Now, you will still the suspension components, for instance the tulips, the drive shaft where the motor attaches to the wheel, and back shafting. But that will be predominantly not by traditional atmospheric quench, it’s going to be done by induction hardening. So, I expect to see an increase in induction hardening or, at least, stay the same, but more atmosphere, traditional atmosphere, endothermic atmosphere and quenching and quenching in a quenchant — that’s going to be drastically hit in the next five to ten years.
DG: So, gears, I assume, cam shafts — we’re not going to see that? Drive shafts to a certain extent, not the same type of drive shafts that you’ve got now, but they’ll be a different type — there will be four independent ones, I suppose. Does the move to EV add anything? Are we doing heat treating of armatures or anything in the motors, motor laminations or anything of that sort? Does it add to the heat treat load?
SM: Certainly, the motor laminations- that requires a special thermal process. It’s not quite heat treating because the thermal lamination is going to require different materials (right, silicon steels). You are also going to see much more, leading into your other question about aluminum heat treating, because the structures are going to be moving in either much higher strength steels or bodies to meet crash tests. You’re either going into aluminum because of lighter weight or for very high performance, you’re going to go into carbon fiber. Carbon fiber will require the resins and the pre-peg will require thermal processing. But that’s more like in an autoclave, like airframers do.
Aluminum will require a different mindset. This will require, and it’s already starting to happen where automotive manufacturers are starting to do aluminum heat treating, and a lot of them are adopting a lot of the aerospace specifications, for good or bad, by AMS 2770 or heat treating recipes. It eliminates a lot of research and development on their part.
DG: Right, you’ve got to stick the AMS 2770.
SM: Or, you can do like the Japanese have done, in many cases. They’re not going to aluminum. What they’re doing is higher strength steel and just making it thinner and they’re going to add using special design steels, much more highly refined grain, you’ve got other stuff in there, you’ve got other stuff, to get the high hardness. Then, what they’re doing is, for instance, they’re forging it at a high temperature, and the Germans are also doing this, too, as part of Audi and Mercedes, is they forge the sheet, they take the forge sheet, they put into a pour compress, they heat it up to the forging temperature, then what they do is then they stamp it into the sheet, into the form, the very complicated form, and then what they do is they quench it while it’s in plaque. In other words, they have all kinds of pulls in the dye and so it’s actually acting like the quench press, in this case, by quench press. So, then they have a fully heat-treated part as it exits the forging press.
DG: And that was steel or aluminum?
SM: Steel.
DG: Steel, ok. High strength steel, specially designed, let’s say, “designer steels,” or whatever. Okay.
SM: So, all it does is once it gets out of the forge press, it’s stamped and goes out. It goes directly into the tempering process. Sometimes it goes directly out without tempering, it gets painted and then puts into a [indiscernable] and that does the tempering operation.
DG: As far as the quenching part, obviously you’re quenching through the dye, as you mentioned, so that’s changing. Is any impact the same type of polymer quenching, I assume?
SM: No, it’s just the mass of the dye. They may use air and the mass of the dye. You know, when you think of it, a dye has to buried large compared to my sheet metal; it’s a thermal mass. So, they’re using the thermal mass of the dye to quench the part.
DG: Which they’re obviously cooling that dye because it’s going to be warming up. Okay, very interesting.
SM: One of the problems is cooling the dye and cooling the dye quick enough, so they have to use all kinds of very special panels, high velocities of water, etc.
DG: Just a quick editorial comment about this: There is a debate out there — maybe you can comment on this if you’d like, Scott — in the “green” world regarding the use of aluminum panels versus steel in the automotive industry with body and white type of panels for cars. Those who are “green” seem to say, “We need to push for aluminum.” But the fact of the matter is aluminum takes a lot more energy and actually has a higher carbon footprint to produce than most steels do when the steels are created. So, it’s an interesting thing that the Japanese and the Germans are moving towards custom design, high strength steels as opposed to potentially aluminum. What do you think?
SM: Well, if you look at aluminum, and it depends on at what point in the process you look at it. If you look at just the overall of aluminum, because of the high degree of recycling of aluminum, we’re not mining anything, we’re not mining bauxite, so all of it goes in and then it’s all ready. All you have to do is melt it and alloy it but grade the alloy.
So, instead of making it with the high energy cost of the bauxite process — which is interesting, some of the cheapest is up in Iceland. It’s just tremendous because of the cost of electricity. It’s really interesting seeing those in Iceland. Anyway, that’s neither here nor there. If you look at the whole process from a cradle to grave aspect, aluminum is very attractive. Steel, on the other hand, while we’re doing a lot more recycling and we’re putting it in instead of the old process where you take the taconite and you make a series of blast furnaces and then you put it into a mixer and then you put it into the open hearth or BOF cast and ingot, etc., now we’re running scrap nearly 100% scrap in an electric arc furnace, put into a caster and out.
So, from electricity required to melt it, it obviously doesn’t take as much electricity to melt the aluminum as it does steel just because the temperature is different. You’re looking at 2700 versus 1200 for aluminum. So, in terms of an environmental impact, you have to look at all the numbers. Aluminum would come out the winner because you don’t have to mine it.
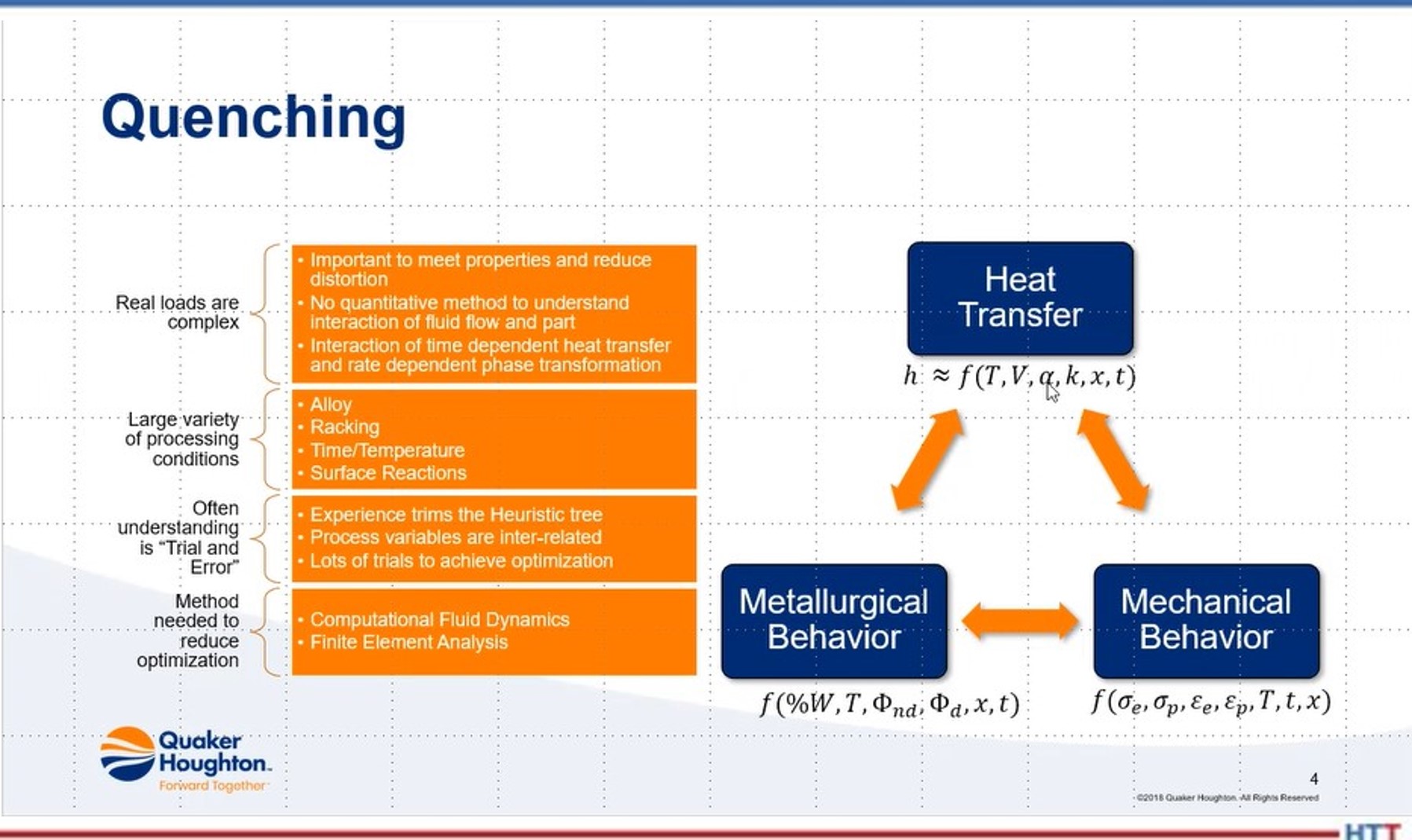 DG: Our next topic I want to talk about with you is simulation and modeling. We’ve talked a bit about that offline, and the developments there. As far as quenching goes, what can you tell us in the quenching world, as far as simulation and modeling? What is happening?
DG: Our next topic I want to talk about with you is simulation and modeling. We’ve talked a bit about that offline, and the developments there. As far as quenching goes, what can you tell us in the quenching world, as far as simulation and modeling? What is happening?
SM: It can be done, and it can be done accurately. But part of that is dependent upon the quality of your materials data. That’s the part. We need to know how that will respond as a function of the constituent of equations within the part. For instance, if I put a stress on it or put a strain on it, what’s the plasticity of the part? How will it perform?
The next thing you have to understand is the quenchant itself. You have to understand the physical properties. Let me share something if I may. Can you see the screen?
DG: Yes, I can actually.
SM: We have to look at the heat transfer. We have to look at the temperature, we have to look at the thermal conductivity, thermal detectivity as well as the position and space (X, Y, Z), as well as time, because you know, obviously it’s a time function. So, we have to understand that within the part.
Now, we also have to do the same sort of thing on the quenchant, but now it’s a function of space on the surface of the part. Now we have to look at velocity, we have to look at surface temperature, velocity, thermal conductivity as well as X, Y, Z, and time.
That’s why there’s been so much modeling and good effect with, for instance, high pressure gas quenching. Because the properties of the gases used are well known, well documented. You just look them up in a table someplace. Quenchants, on the other hand, the quenchant suppliers have done a lousy job of documenting the thermal properties. That’s starting to change. So, that’s one of the problems that you see is that the thermal properties of the quenchant are not well established.
The second thing is, is looking at the boundary conditions of the part is that changes as a function of position and agitation — the agitation rates can change around a part. If I look a part, the quench rates change as a function of velocity. Well, the suppliers have not done a real good job of characterizing their quenchants as a function of velocity. That’s a problem, which is getting worked on.
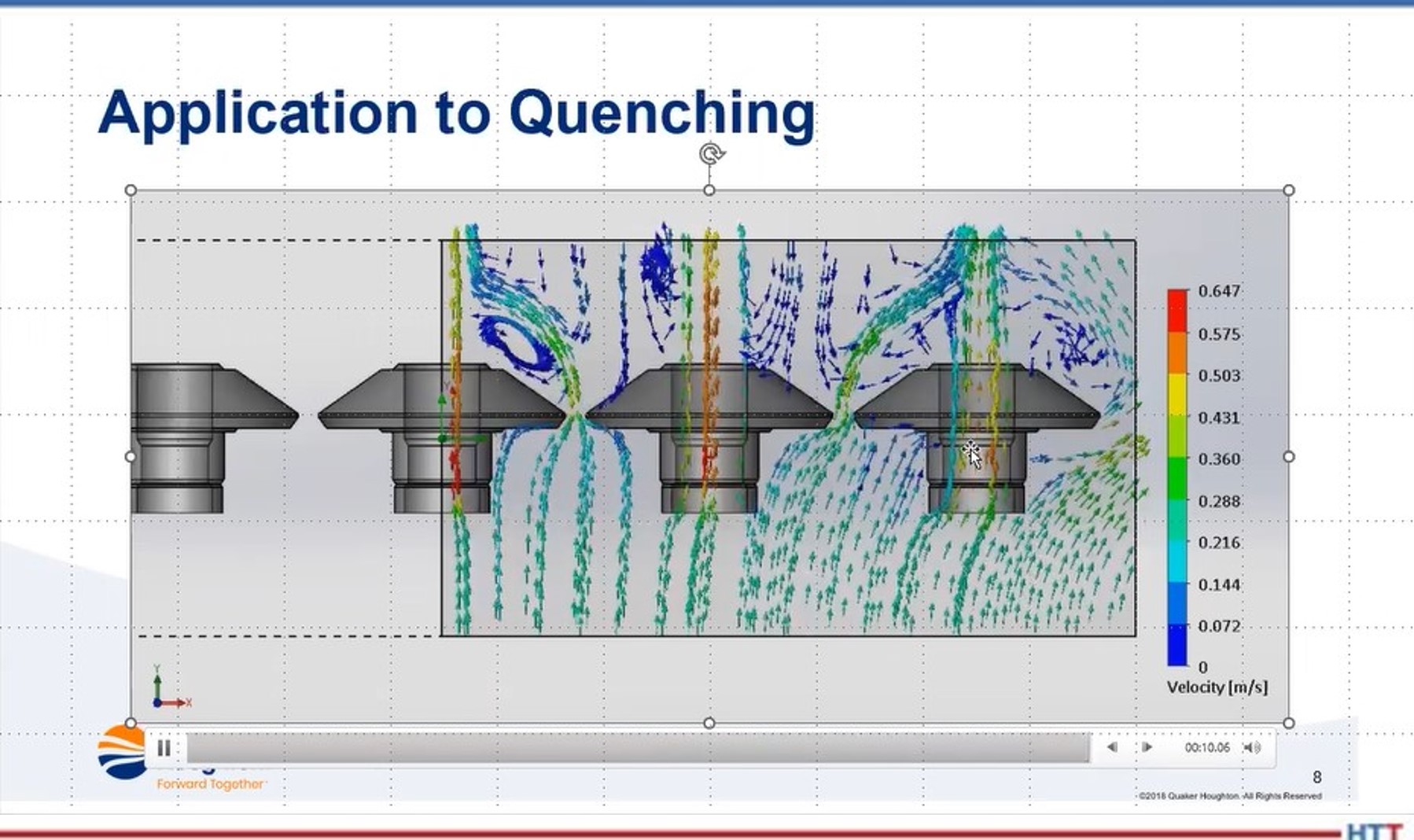 In terms of the simulation, it can be done if you’ve got good boundary conditions. The boundary conditions being the stuff on the outside of the part and the stuff inside the part. Once you do that, and you can do this with either using something like computational flow dynamics and then applying that as whatever velocity heat transfer coefficient that you get out of that and apply to the boundary of the part, then you can use a variety of different software programs, such as Dante or SIMHEAT — both of those are good, just a difference in their material databases. Each will give similar results but it’s a function — garbage in, garbage out. You have to have good material properties and good boundary conditions. If you have those, then you can get a reasonable result. But, if you don’t, you’ll just get garbage results.
In terms of the simulation, it can be done if you’ve got good boundary conditions. The boundary conditions being the stuff on the outside of the part and the stuff inside the part. Once you do that, and you can do this with either using something like computational flow dynamics and then applying that as whatever velocity heat transfer coefficient that you get out of that and apply to the boundary of the part, then you can use a variety of different software programs, such as Dante or SIMHEAT — both of those are good, just a difference in their material databases. Each will give similar results but it’s a function — garbage in, garbage out. You have to have good material properties and good boundary conditions. If you have those, then you can get a reasonable result. But, if you don’t, you’ll just get garbage results.
DG: As far as simulation goes, obviously it’s something that can be done. Do you see the use of it growing significantly over the next 5-10 years and, if so, any particular areas do you see it growing? I’m assuming it’s going to be in high value parts, right? You’re probably going to see it more there than in your nuts and bolts.
SM: I see it more in the higher value parts. And also, induction hardening. Let me explain: One, in the high value parts because they want to be able to characterize the parts. Either as, “Oops, I sent this part out and it cracked, what happened” as an analysis tool to prevent or to explain why something broke. I see this occurring more in the automotive world at the OEM level. You see some of it in the second-tier aerospace where they’re trying to understand to reduce residual stresses, reduce distortion. At the commercial heat treat? No. They just get paid to quench the part and shove it out the door.
DG: Is it genuinely accessible today? You mentioned Dante and things of that sort. I know Quaker Houghton probably is, but are most of the quench companies working with modeling or is it not that commonplace?
SM: It’s not that common. Part of it is because, you know, the quenchant business is a very competitive business. It just is. A lot of people look at it as strictly a commodity. Quite frankly, we’ve lost sales, I’ve lost sales, over a penny a gallon. And so, one of the things that’s very difficult, and it’s more difficult for the salespeople is to look at the value ad and that value ad can either be we’re not the cheapest quenchant out there. We’re the Cadillac, we’re not the Chevy. So, to justify that higher price (and my salary), we have to sell the value ad, and that value ad can be help with making sure that when I quench my parts in it, I’m going to make properties.
For instance, most quenchant suppliers do not have a metallurgist. One, metallurgists are hard to find anyway, so they’ll get a materials science person which may or may not be exposed to heat treating. So, they have to help them understand whether or not they’re going to make parts. In other words, to mitigate the risk in changing to another quenchant. The value ad is the back-up support from the metallurgical point of view. That’s help understanding, not only just the chemistry of the quenchant and what it does, but what happens to the part. Why is my part stained? Why did my part crack? Or why did my part work this way as opposed to that way? How can I approve the residual stress state in that part? How can I reduce distortion? How can I achieve better properties? Those are the things that we can help with.
Some of the other suppliers can also do it, but they’re not doing using modeling or using computational flow dynamics or using the modeling program, they’re doing it based on their experience. It’s something I do too, but I can do that with the modeling and my experience to get it even closer.
Did that answer your question?
DG: Yes. Basically, I was just trying to get a sense from our listeners, many of them are going to be manufacturers with heat treat in-house, “captive heat treaters,” as we call them. I’m just curious how accessible it is. Is it something they can call today and say, “Can you help me with this, and can we model it?” It sounds like, “yes” but not with all quench suppliers, but it is possible.
SM: There are also consultants out there that can do it.
DG: Speaking of green, speaking of money, Quaker Houghton, several years ago, probably three or four years ago. . .
SM: Three years, next month.
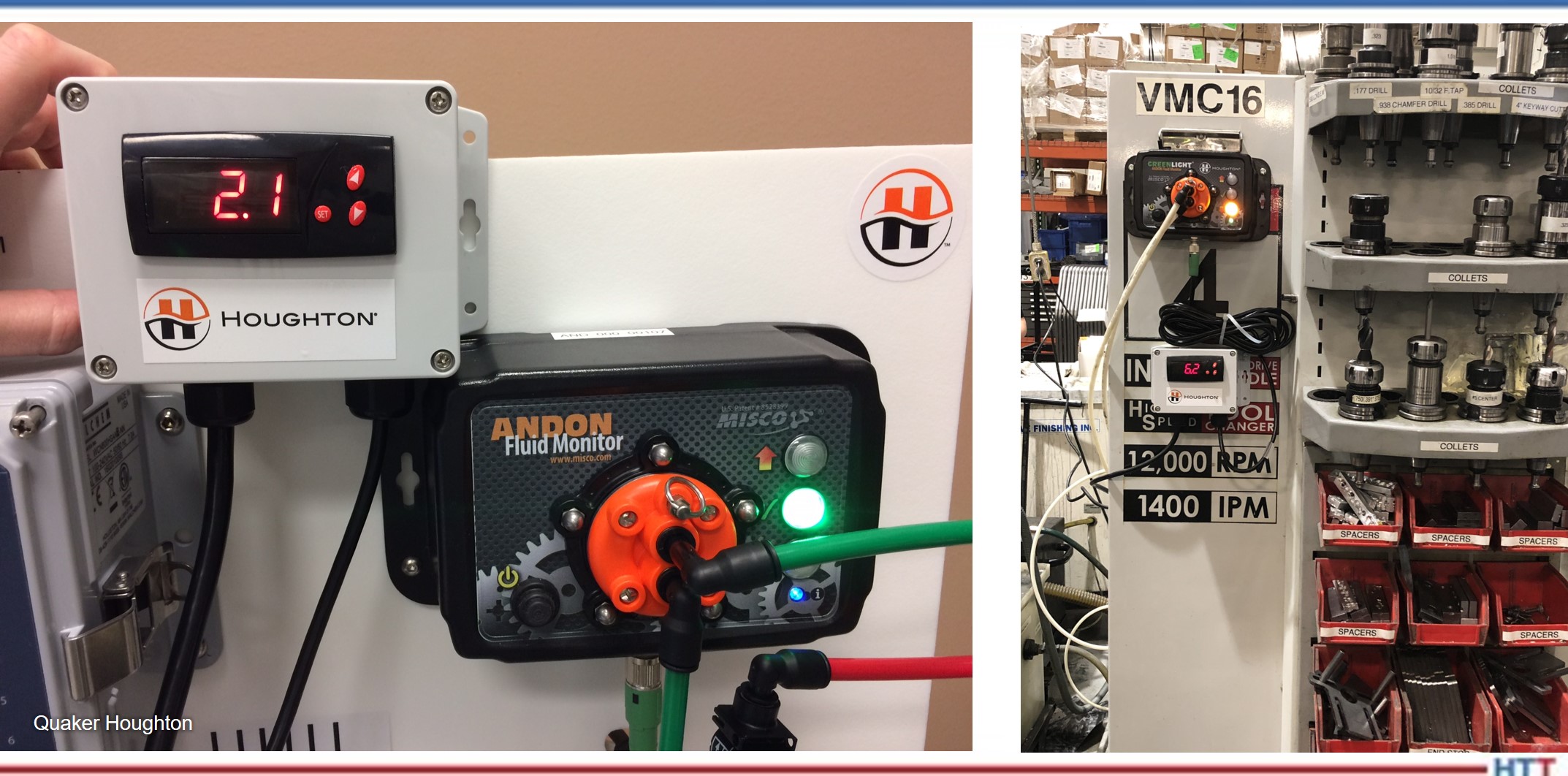
DG: . . . came out with this product called Greenlight Unit and I’ve been wanting to talk to somebody over there about that. From a 30,000-foot view, what is it, why does it work, why should people care about it?
SM: What the GREENLIGHT unit is, at it’s very simplest — you’re measuring something and that measuring something could be, for instance, polymer concentration using [indiscernible]. You’d be measuring ph. You could be measuring some other physical property. You tell the unit — these are the ranges that I want to use. You can use it to computer interface or PLC interface, and I set this box on, for instance, my induction hardener which is very common. I have a concentration range for the polymer quenchant. If I go below that it puts a big red flag. If everything is good, it waves a green flag. If it’s either too high or too low, it waves a red flag and says, “pay attention.” Now, that red flag can be either I could add water or add polymer and I could tell either a person to do that, you know, “Operator, come and do this for me” or it can tell a PLC to actuate a pump — either add water or to add polymer. All automated, don’t have to pay attention to it.
DG: And that works, not just on induction equipment, just to be clear. You can do this on quench coolant tank or whatever.
SM: Yes, absolutely, anywhere. I can put it on polymer quenchant, for example. Most commonly, it is being used on induction. In fact, it’s standard on some of the induction hardening equipment.
DG: So basically, just a simple human-machine interface or human-quench fluid interface is going to tell you whether it’s within spec or not and if it’s not in spec, the green light goes out and the red light comes on.
SM: And some alarm comes on and some enunciation, whether it’s visual or audible or both.
DG: And you either fix it manually or you’ve got it programed so that a PLC can make whatever adjustments.
SM: You can contact those so that you can tell a PLC to do some action.

DG: Let’s hit one other main topic before we wrap up today. You’ve already kind of hinted at it, but I think that it’s something that’s important. We’ve talked a lot about “brain drain” in the industry and the fact that, and you and I actually spoke off-line not too long ago about, metallurgy programs versus material science programs and the fact that sometimes material science graduates don’t necessarily have a full grasp on what metallurgy is and how it works. . . .
When companies that are manufacturers with their own in-house heat treat are needing help, how are they going to get training? Where can, in fact, they go to get questions answered and things of that sort. And how bad is that problem?
SM: One, it’s a global issue. Metallurgy is kind of like a forgotten science. I was one of the last at Ohio State to actually graduate with a metallurgy degree, metallurgical engineering. After that they changed to material science.
The reason is because one of our illustrious funding [parameters] for grant-funding says: We already know everything there is to know about heat treatment metallurgy; we need to be focusing our energies on nano-this or green-this or additive manufacturing or whatever kind of buzz word. In other words, I’ll send something in, toss in those buzz words and you can get a grant. In other words, it’s because the universities are chasing the government cheese when, really, what the industry needs is people who have a strong grasp of the metallurgy of something. For instance, when I went to school, back in the dark ages (about 1980), back when we still used slide rules (I still have mine), we actually had whole courses, multiple semesters on heat treating. How does a steel react when I change the quench rate? We have the different microstructures you get. Looking at the microstructure, what do we get?
Now, with a material science degree, what we were exposed to in multiple semesters, they may get mentioned in a single lecture.
DG: And spend the rest of the time talking about plastics, polymers, composites and high-faulting new stuff, which is important, but. . . .
SM: Just to give you an idea: I had a customer, and they were having, roughly, 95% cracking. They asked me to help. They’re using our quenchant. What they were doing is that they were taking the parts and they were putting them into the high temperature in the austenizing furnace. They would then quench them into our polymer quenchant, and these were parts like 4340, big parts. They only had one furnace. So, what they would do is after they quenched it, they’d take up the parts then they would put them outside in the snow so they could let the furnace cool down so they could then temper them. Usually, it would take overnight. But when they would come around the next morning, all these big, expensive, large — and we’re talking several hundred-pound parts — were sitting there in multiple pieces because of quench cracking. They wanted to understand why this was happening. So, I go in there and I meet and talk to their metallurgist, and I said, “Ok, the problem you’re having is an issue with quench cracking which is due to transformation martensite, and you need to get rid of the residual stresses by putting in to temper immediately. The metallurgist looked at me and asked me, “What’s martensite?” I had to control my . . . yeah. And I asked her, “Where did you go to school?” She went to Carnegie Mellon.
DG: Not that it’s not a good school; your point being they’re not covering the metallurgy that they need.
SM: I looked at her and I said, “I know a lot of the professors there. In fact, I flunked out of Carnegie Mellon.” You know, I got lousy grades, I flunked out of Carnegie Mello. I was accepted and then flunked out, so I know! I mean, Metallurgical and Materials Transactions A is by Dave Laughlin who is at Carnegie Mellon. He is a wonderful person; I think he may have retired now. He was a wonderful professor, and he gave me my first metallurgy program. He was also very supportive of me throughout my career. But I looked at him and said, “As I recall, we were taught these courses, I had. . . I mean we were taught these courses.” I mean we had Massalski, Laughlin, I had a whole bunch of people that were well up in the [field]. She looked at me and said, “Well, it was a material science degree, and I took the ceramic option.” So, anyway, we had to go through and do all the training, what’s required and all that stuff. We got it and so we understood what was going on, we understood the ramifications of different quench rates and got that all resolved.
Then I talked to this When I was working on my. . . . Afterwards, I talked to one of my professors who has since passed away at University of Missouri Rolla (or now known as Missouri Institute of Science & Technology), and he said that’s unfortunately truth. If you want somebody that’s knowledgeable in heat treatment, don’t hire a material science person, hire a mechanical engineer because at least they will be exposed to it.
DG: That’s a good point. It’s possible that the mechanical engineers are going to have more exposure to, at least, the effects of heat treat and understand heat treat more than maybe materials engineers do who may have one course. You mentioned before, Scott, that there are only a couple of schools in the U.S. now that still maintain an actual metallurgy degree. Do you recall who they are?
SM: Yes. I believe the University of Missouri Rolla (Missouri Institute of Science & Technology) in beautiful and scenic Rolla, Missouri. There is the University of Arizona, but I believe they are focused strictly on, mostly, mining. . .
DG: Yes, because there’s a heavy metallurgy emphasis in mining, as well.
SM: . . . There is the University of South Dakota and maybe the University of Idaho, but I’m not sure on that one.
DG: The Colorado School of Mines? I think they, at least, used to.
SM: Yes, they still do. But that’s four colleges.
DG: I guess an application here is for companies who are looking to hire people to help them with metallurgy because what we’re talking about here is training and getting the brain-drain, is to be very careful who you’re hiring and where they came from. Not to say that all materials engineers are not worth their salt, because that is not the case, but you need to ask the question: “How much exposure, what has been your experience in metallurgy, specifically?” I think that’s the point.

SM: And I’ll tell you what. The industry right now is a bunch of old guys. We’re retiring. I’m going to be retiring probably in the next up to three years. But if you look at other people in the world, we’re all getting up there, and the young people to replace us will have to be knowledgeable, otherwise we’re going to repeat all the same mistakes all over again.
DG: Well, I want you know, there are a lot of young people coming up in the industry, right there, 40 Under 40. There are some good, good people. It’s amazing. But your point is very well taken.
SM: And one of those 40 Under 40 has been brought along. Sergio.
DG: Sergio, wonderful, wonderful.
SM: That said, somebody that is very knowledgeable in heat treatment, is still going to be needed —whether you’re doing for production of gears, not necessarily for transmissions, but gears or wind turbines. Heat treatment of turbine blades, heat treatment of . . . whatever. Somebody who’s knowledgeable in heat treatment, a young person, will be able to write their own ticket.
DG: I agree with you!
SM: One of the beauties of heat treatment that I’ve had is I’ve never had to worry about losing my job, I’ve never had to be worried about being laid off, and I’ve been through some ugly layoffs. When I was at McDonnell Douglas, we had 64,000 people at one time; the next morning we had 30,000. In one day, they laid off 35,000 in one location. So, I’ve never had to worry about being laid off. I’ve never had to worry about — if something happens, will I be able to find a job? I’ve never had that issue.
DG: It’s never been an issue for you. That’s great.
SM: And I think that that will be true of any young person in heat treating. You’ll always be able to find a position.
DG: That’s great, Scott. I appreciate it. Just to wrap this one little segment up as far as training goes, the fact of the matter is, if you don’t have in-house resources to help you understand heat treating and/or the quenching aspect of it, I think, point being, there are consultants out there that can do it, there are quench companies like Quaker Houghton, for example.
SM: And there are heat treating societies, for instance, ASM heat treat society. Since this is global, all of the heat treating societies, whether it is the Chinese heat treating association, the Chinese heat-treating society (there are two of them), ASMET which is the Austrian, IWT which is the German, the Italian heat treat society, the Czechoslovakian, Indian heat treat society (which is actually part of ASM) — all those societies have their own training programs and they’re good. I taught some of them and other people have taught. Take advantage of your local heat treating society. And do the training of your own people. Or you can use consultants.
DG: Right. And I was going to say to anybody listening, if they need help finding those resources, you can feel free to call us. I’m sure that Bethany will put some information in this podcast about how you can get ahold of us to help. If nothing else, we can put people in touch with you, Scott, which leads me to the final question: How much information are you willing to give away as far as people contacting you. And don’t worry, you’re probably not really allowed to retire, so even if you do, these people will find you. How can they contact you?
SM: Well, you have my email address — scott.mackenzie@quakerhouton.com. Right now, I’m not taking any consulting positions. I get asked routinely. Part of that is because it’s a conflict of interest with my existing job. If you’re using our quenchants, I can help you. Or, if you’re looking to use our quenchants, I can help you. And that isn’t just choosing a quenchant. Obviously, I can help you select a quenchant if you’re unhappy with your existing product. But I can also help you minimize distortion, better reproduce better properties, whether that’s now we do do a company can come to us and ask for CFP modeling of a quench tank — we can do that. Or we can do that as part of the modeling of the part, we can do that. And we can do it and tie them together, as best we can, depending on the position of the quench tank, and we can do that on as-needed basis. So, I can help you in that fashion. But there are also other people out there — Andy Banka at Airflow Sciences, which can do CFP work; Dante Technologies; TRANSVALOR in Europe and in the U.S. can also do stuff. We happen to work with TRANSCALOR. They can all do that, and they can do it for a consulting fee.
So, it can be done. When I figure out when I’m going to retire, then I’m going to try and figure out what I’m going to do after that.
DG: We’ll find you, don’t worry; you won’t be able to hide.
SM: That’s what I’m afraid of.
DG: Exactly. Very good, Scott. I appreciate it. Are there any closing comments you’d like to make? Is there anything we missed that you’d want to include? I think we’ve hit on most of the major stuff we were thinking about.
SM: I think probably the biggest thing is encourage your young people to go to conferences, and I’m not just talking about where they’re laying out a whole bunch of equipment. Not just an exhibition so you can look at equipment. They need to go to the events so one, they can meet other experts, so they can be educated, and I’m not just talking about taking an ASM course; I’m talking about going to the conference, being able to ask questions of other experts as well as talk to their peers. What are the problems their peers are having? The point is, it’s likely the same sort of problem. And be able to expand the horizon by seeing the conference, the conference proceedings, etc. Encourage them to go to those sorts of things. And also submit papers, etc. because that’s the only way they’ll grow. And that’s what you want, you want the people to grow within the organization, and encourage them to grow within the organization so they become more of a value to that organization.
DG: Yes. There’s no better way to learn than to teach. Once you decide you’ve got to teach, you’ve got to learn the stuff.
Well, you’ve done a great job of that over the years, Scott. I know there’s many, many people in the industry who have appreciated your expertise and we certainly appreciate you being with us here today. Thank you very much for your time and we’ll look forward to talking with you again. Don’t retire too soon — we’ll need you here, so stick around!
SM: Ok. Thank you.
For more information, contact:
Website: quakerhoughton.com
Contact Scott: scott.mackenzie@quakerhoughton.com

Doug Glenn
Publisher
Heat Treat Today
To find other Heat Treat Radio episodes, go to www.heattreattoday.com/radio .
.
Search heat treat equipment and service providers on Heat Treat Buyers Guide.com
Heat Treat Radio #79: All Things Auto Industry Quenching with Scott MacKenzie Read More »















 DG: I should congratulate you on that degree, by the way. I know a year or so ago, you were still working on that, so that’s great!
DG: I should congratulate you on that degree, by the way. I know a year or so ago, you were still working on that, so that’s great!