![]() When was the last time the parts washer was cleaned? For many heat treaters, answering this question and keeping data on cleaning schedules and outcomes may not be at the top of their priority list. Learn how a data-driven approach to cleaning heat treated parts can have an impact well beyond the cleaning phase.
When was the last time the parts washer was cleaned? For many heat treaters, answering this question and keeping data on cleaning schedules and outcomes may not be at the top of their priority list. Learn how a data-driven approach to cleaning heat treated parts can have an impact well beyond the cleaning phase.
This Technical Tuesday article, written by Greg Steiger, senior account manager at Idemitsu Lubricants America Corp., was first published in Heat Treat Today's August 2022 Automotive print edition.

Senior Key Account Manager
Idemitsu Lubricants America
Introduction
For many years heat treaters have virtually ignored their washers. It was not uncommon for these washers to be dumped and recharged whenever someone thought about it. Often the question “When was the last dump and recharge?” was met with the “I don’t know” shoulder shrug or “When the parts were dirty.” So why do parts need to be cleaner than ever before? The easy answer is because it is what customers are demanding. The more difficult answer is because as quality standards have improved over the last several decades, the need for parts with tighter tolerances has also increased.

Many readers will wonder what part cleanliness has to do with tighter tolerances. The answer is the quench oil residue that was once acceptable to leave on the parts affects the tolerances of the part. For example, a buildup of oil in the threads of a part will have an impact on how the part threads into its mating part. Cleanliness affects post heat treat processes such as plating and painting as many residues cannot be plated or painted over. Part cleanliness also influences the shop environment in a heat treat operation. A clean, oil free part will not produce smoke in temper like a part with oil residues will.
Furthermore, when asked how the washer was recharged the typical answer was to drain the cleaner solution and then replace the cleaner solution with fresh water and enough cleaner to bring the concertation to the desired level and then restarting the washer. There was virtually no thought to removing the sludge that had built up over the years since the washer was last thoroughly cleaned. Little time was spent mucking out the sludge, and even less time and thought were expended on determining if the spray nozzles were clogged or properly aimed at the load. When energy, labor, cleaner chemistry, and disposal costs were all very low, this was the typical method of operating washers.
Now (with labor, disposal, energy, and cleaner prices all increasing along with the nationwide labor shortage) is the time to change those old habits and recognize the preclean and post quench washers are two ways to improve part cleanliness and the bottom line. The method of change for these habits is to allow data to be the guide in operating the washers in a heat treatment operation. The data will determine what the optimal concentration range is to obtain the cleanest parts. The data will show when the soil loading in the washer is too high. The data will reveal the maximum tank life for the cleaner solution. In other words, data should be used to maximize the efficiency of the washers.
Basic Cleaner Chemistries
The term alkalinity in its most basic description is a pH above 7.0. At this pH, the cleaner efficacy is improved as is the overall rust protection of the parts in the quenched load and any mild steel used in the washer construction. All alkaline cleaners share several types of common raw materials. They are alkaline builders, surfactants, corrosion inhibitors, and sequestering agents. However, where the
cleaner chemistries differ is in the types of alkaline builders used to create the alkaline pH. Many older formulations and less expensive products use caustics, carbonates, phosphates, and silicates as their alkaline builders.
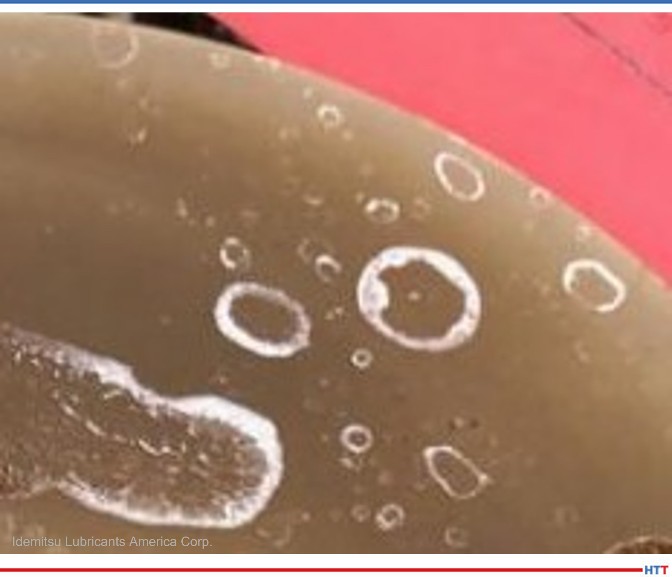
Source: Idemitsu Lubricants America
While these are now commonly used in the liquid form, they are all powder based. The biggest issue with using powder based alkaline builders lies in the residue they leave behind when the water evaporates. These residues are the hard white residues seen in Figure 1.
Additionally, when the water evaporates from cleaners using these alkaline builders the residue can clog the spray nozzles within the washer cabinet. More recent formulations have begun using a product called an amine as an alkaline builder. Amines are liquids mixed with water. Therefore, when the water evaporates a liquid is still left behind. The film from an amine is more uniform, does not leave a powdery residue, and does not clog the spray nozzles in the washer cabinet. Additionally, amines have better buffering capabilities and help keep the pH of the cleaner in the mild pH range of between 9.0 and 10.5. When water is sprayed on a warm piece of steel, the water beads up and forms droplets.
The purpose of the surfactants in an alkaline cleaner is to prevent this from happening. The surfactants help the cleaner to wet out over the load more evenly. Surfactants also assist in the cleaning process by providing detergency to the cleaner. To provide short-term indoor corrosion protection, alkaline cleaners also have a short-term corrosion inhibitor formulated into the cleaner. This short-term protection is only intended to provide work-in-process protection. This protection is typically no more than a few days of protected indoor storage. Lastly, sequestering agents are used to allow the alkaline cleaners to be used in hard water. The sequestering agents chemically react with the minerals in hard water preventing them from precipitating out as hard water soaps and salts.
Alkaline cleaners can also be distinguished by those that emulsify the oils they remove and those that separate the oil they remove. In a typical dunk spray post quench washer, the load enters the washer and is lowered into the cleaner solution where the solution is agitated. The agitation allows the surfactants or detergents to provide the cleaning. During this period of agitation, the cleaner and quench oil combine to form a mechanical emulsion and potentially, a chemical emulsion. (The difference between a mechanical and chemical emulsion is a chemical emulsion is a more permanent emulsion and a mechanical emulsion stops when the mechanical agitation stops.) Once the mechanical agitation stops, a still period, or dwell, should then occur. This will allow the mechanically emulsified oil to separate from the cleaner solution. After this dwell period is over, an air knife or a set of nozzles will blow the oil layer into a separate chamber where the oil can be skimmed and removed from the cleaner solution. The elevator then brings the load up and out of the cleaner solution and into the spray cabinet. At this point it becomes highly imperative as much oil as possible is removed from the top of the cleaner solution. If the oil is not removed, the elevator will simply bring the load through a layer of oil, which is redeposited throughout the load. Once the load is in the spray cabinet, the cleaner solution is pumped through the spray nozzles onto the load. This spraying action is to remove any lingering soils and remaining oils. The solution pick-ups for these nozzles are typically in the middle portion of the dunk tank.
Designers of the equipment chose this spot because any free-floating oil will not be picked up and sprayed through the nozzles. For cleaners emulsifying oils the cleaner and oil emulsion is then sprayed and redeposited back onto the load. This will create issues in the temper where the water evaporates, and the oil left behind will create smoke and other vapors.
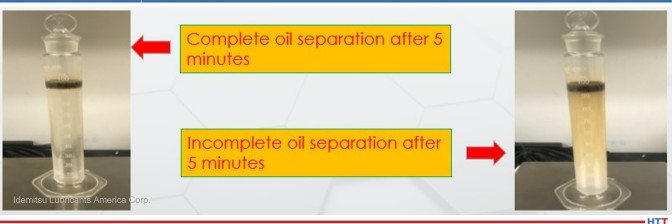
made of each cleaner to test oil separation abilities.
Source: Idemitsu Lubricants America Corp.
For cleaners not emulsifying oils, the oil is not redeposited on the parts and the smoke and other vapors from emulsifying cleaners are greatly reduced or eliminated in temper. Figure 2 shows the difference between emulsifying and non-emulsifying cleaners.
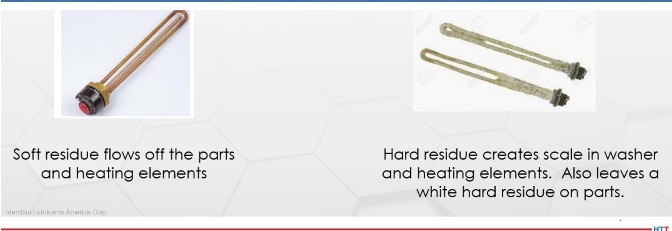
While the source of alkalinity does not create smoke and other vapor issues in temper, the alkalinity source does create issues in temper and in the spray portion of the washer. In the temper, cleaners using alkaline builders such as caustics, carbonates, phosphates, and silicates will leave behind a white powder residue as seen in Figure 1. This residue is caused when the water in the cleaner solution evaporates and leaves behind the powder of the alkaline builders. Water evaporation in cleaners with powder alkaline builders will cause spray nozzles to clog and heating elements to foul. Cleaners using an amine as the alkaline builders do not have these issues. The difference in heating elements can be seen in Figure 3.
Selecting a Cleaner
Source: Idemitsu Lubricants America
The proper selection of a cleaner can be the difference between a highly satisfied customer and a completely dissatisfied customer. The requirements for a cleaner are as follows:
• Part cleanliness that exceeds customer
expectations
• Long sump life
• No residue
• Ability to split quench oil from cleaner
• Rust-free parts
• Low foam
• Low to moderate pH
• Hard water stability
When selecting a cleaner, a heat treater typically has two opportunities to influence the overall part
cleanliness. The first opportunity lies before the heat treatment process begins with a precleaning step. The second opportunity is with the post quench cleaning operations. When choosing a cleaner for these operations it is important to know what soil will be removed during the cleaning. The answer to the post quench cleaning is obvious, a quench oil. However, the soils on the parts incoming to the heat treatment process vary greatly. These soils may include oil and water based rust preventatives, water soluble coolants, cutting oils, and mill oils.
Typically, the soils removed before the parts are placed into the furnace are easier to remove than the quench oil from the post quench washer. This allows for the same cleaner to be used in both operations. By using the same cleaner in both preclean washer and post quench washer, heat treaters don’t have to worry about purchasing two different cleaners or have the concern of mixing the cleaners by placing the incorrect cleaner in the wrong washer system.
Once the soils to be removed have been identified, the next criteria to look at in selecting a cleaner are the operating temperatures of the washer, the pH of the cleaner, and foaming characteristics of the cleaner. Typically, the foaming characteristics and the operating temperature of the washer are directly related.
The type of surfactants or detergent additive used in alkaline cleaners have a property called the cloud point. At operating temperatures below the cloud point, the cleaner will form a dense and heavy foam that inhibits the cleaning efficacy of the cleaner. At operating temperatures above the cloud point, the surfactants are soluble in water and work as detergents and do not create foaming. An operating temperature of 140°F–160°F is the ideal operating temperature to remain above the cloud point, maximize the efficacy of the detergents, and minimize foaming tendencies of the cleaner. The cloud point phenomena can be seen in Figure 4.

Source: Idemitsu Lubricants America
The higher the pH the easier it is to clean many soils from the parts. The pH of a cleaner plays multiple roles in the parts cleaning process. A pH above 8.0 also helps provide corrosion protection on mild and carbon steels. However, as the pH climbs, skin sensitivity becomes an issue. At a high caustic pH such as 12 or above chemical burns on skin can occur. At lower pH levels of between 9 and 10.5, such as those provided by amine-based chemistry, skin sensitivity is greatly reduced.
Another advantage to amine-based chemistry lies in the lack of a perceptible residue that is often seen on parts after temper or around the washer itself. Figure 5 shows a typical part residue after temper from an emulsifying caustic cleaner. Figure 6 shows the residue found on a washer using a caustic cleaner.
Source: Idemitsu Lubricants America
Source: Idemitsu Lubricants America
In addition to leaving the residues seen in Figures 5 and 6, caustic cleaners also have the potential disadvantage of clogging spray nozzles when the water evaporates leaving behind the same type of residue in the spray nozzle. The clogged spray nozzles will then reduce the efficacy of not only the cleaner, but also the oil skimmer as well as the spray nozzles that are used to push the floating oil into the quenchant tank where floating oil is removed via an oil skimmer.
A cleaner should be compatible with hard water. In many areas the aquifers and wells where water is drawn from contain high amounts of minerals and salts. These hard water minerals and salts exacerbate any residue issues and create an ideal environment for rust and corrosion to begin. If the minerals and salts are left unchecked, they will eventually form chloride ions and mini voltaic cells. These mini voltaic cells are the beginning stages of the corrosion process. The sequestering agents in an alkaline cleaner will chemically react with the minerals and salts thereby not allowing the free chloride ions and the mini voltaic cells to form.
Using Data To Efficiently Operate a Washer
There are many reasons heat treaters dump and recharge their parts washers. The most common reasons typically are: “we dump the washer once a month because we always have”; “we dump the washer whenever the parts get dirty”; or “we never dump the washer.” Very infrequently is the answer “the soil loading is too high.” That is because to know what the soil loading is, the washer has to be operated by using data. Using data, heat treaters can optimize the efficacy of the cleaner solution, maximize the dump interval of the cleaner, reduce the amount of sludge in the washer, and lessen downtime.
The key in establishing a dump cycle is to know when the cleaner has reached its soil loading limit. Typically, this is around 2%. Soil loading is the amount of soil that is mixed in with the cleaner. The soil consists of a mixture of the soils removed, dissolved salts, and soaps along with anything else that makes its way into the washer. The 2% limit will be reached quicker in the post quench washer than in the preclean washer as more soil is removed in the post quench washer. In addition to soil loading, the proper data approach should also include the cleaner concentration by an alkalinity titration, concertation by Brix, tramp oil, cast iron chip rust test, and chloride level.
A brief explanation of each test and the reasons for performing the test are individually listed below.
pH
A good pH range is between 9.2 and 10.5. Within this range, most people coming into contact with the cleaner solution will not have an issue with skin sensitivity. At a pH above 10.5 skin sensitivity dramatically increases. As the pH begins to trend lower and eventually becomes acid below 7, the corrosion protection properties of the cleaner decline.
Concentration by Brix
This test measures everything that is dissolved within the cleaner solution. This includes salts, soaps, and removed soils. The Brix% is measured with a handheld refractometer reading in Brix%. The Brix% is then compared to a chart specific to the cleaner being tested. The Brix% will typically be higher than the concertation when tested via an alkalinity titration as the Brix% captures the amount of cleaner dissolved in water, along with salts, soaps, and removed soils. The concertation limits for the Brix% should have a maximum no more than 2.0% above the concentration by alkalinity.
Concentration by Alkalinity
This is a titration that can be performed in a lab or at the washer. A weak acid such as 0.1N HCl and an indicator such as phenolphthalein is used. The method and concentration multiplier depends on the specific cleaner used. Many methods count drops of acid used, while others use milliliters used to change the color of the indicator. The supplier of the cleaner will likely provide an initial concentration test kit and instructions on how to use the kit. A good concentration range for a preclean washer is between 2% and 3% and a post quench washer should have a concertation range of between 4% and 5%.
Soil Loading
The difference between the concentration by Brix% and concentration by alkalinity is the soil loading. This value should not exceed 2%. When the soil loading exceeds 2% it is time for a dump and recharge of the cleaner solution.
Tramp Oil
A tramp oil test measures the ability of the skimmer to effectively remove the quench oil from the top of the cleaner. This test is simple to run and can be run by most heat treaters. Simply fill a 100 ml graduated cylinder with the cleaner solution from either the preclean or post quench washer and allow the cylinder to stand idle for 20 minutes. Then simply read the amount of oil that has separated from the cleaner. A maximum level of 2% tramp oil shows the oil skimmer is effectively removing the tramp oi from the cleaner.
Cast Iron Chip Rust Test
Running the cast iron chip test requires dry machined cast iron chips and is best left to your cleaner supplier. The purpose of running the cast iron chip test is to ensure the corrosion protection formulated into the cleaner is not being depleted. This test uses a scale published by ASTM with a rating system of 0 to 5, where 5 is the worst and 0 is the best. To successfully pass this test a result of no more than 1 should be achieved. It is important to remember, cast iron chips have more surface area than a steel part and cast iron is also more porous and prone to oxidation than steel. Therefore, a test result of 1 is not a reason for concern.
Chloride
The chloride test is another test that is best left up to your cleaner supplier because the easiest way to test is through expensive instrumentation. The purpose of testing for chlorides is to prevent the situation for a mini voltaic cell to form. If the chloride level exceeds 150 ppm in a cleaner solution a mini voltaic cell can form and the corrosion process begins. As this process begins, the pH will begin to fall as will the corrosion protection of the cleaner.
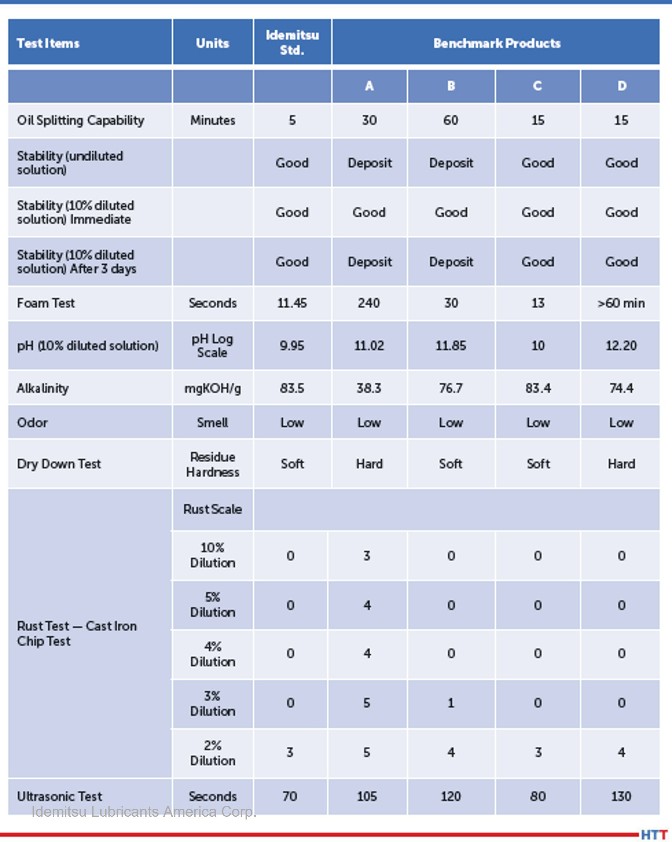 In Table 1 several commercially available cleaners were tested and evaluated using the criteria above. The cleaners tested were both those that emulsified oils and split the oils. Testing also includes both amine-based and caustic-based cleaners.
In Table 1 several commercially available cleaners were tested and evaluated using the criteria above. The cleaners tested were both those that emulsified oils and split the oils. Testing also includes both amine-based and caustic-based cleaners.
Discussion
Imagine if the dump cycle went from four weeks for a post quench washer to 10 weeks for the same washer by using a data-driven approach described in this paper. The savings would not only be in the cost of the cleaner used but would extend to less downtime and more efficient use of maintenance as employees no longer need to clean out a washer every month. Customer expectations for clean parts have changed over the past years. What was acceptable as little as five years ago is no longer acceptable today. What hasn’t changed is the way preclean and post quench washers have operated. While it is difficult to assign an economic value to exceeding the cleanliness standards of customers, it is not difficult to assign an economic value to parts not meeting your customer’s standards. That economic cost can be as high as lost business. By using a data-driven approach the decisions made in how to operate a washer are no longer kneejerk reactions. Instead, these decisions have a historical data-driven approach to them.
About the Author: Greg Steiger is the senior key account manager of Idemitsu Lubricants America Corp. Previously, Steiger served in a variety of research and development, technical service, and sales marketing roles for Chemtool, Inc., Witco Chemical Corporation, D.A. Stuart, and Safety-Kleen. He obtained a BS in chemistry from the University of Illinois at Chicago and recently earned a master’s degree in materials engineering at Auburn University. He is also a member of ASM. Contact Greg at gsteiger.9910@idemitsu.com.
 Find heat treating products and services when you search on Heat Treat Buyers Guide.com
Find heat treating products and services when you search on Heat Treat Buyers Guide.com










